The End of the Transactional CRO?
Posted By Jon Lavietes, Monday, October 19, 2020
Clinical trials have undergone a bit of a transformation in recent years. Much like what has happened with pharmaceutical manufacturing operations over the last decade and more, clinical trial site management is becoming less transactional and more collaborative. Rather than employing a CRO to manage an individual trial with a firm start and end date in a vacuum, pharmaceutical companies and clinical trial site operators are beginning to engage in open-ended, long-term engagements in which the latter works with Big Pharma sponsors on multiple trials over time. In other words, site sponsors and clinical trial sites now apply a shared vision, open communication, and a dedicated infrastructure to a portfolio of trials, and continually refine their methods using lessons learned from each initiative.
Dimitri Fillos and Jessica Piggee, senior alliance and partnerships leaders at Genentech, pulled back the curtain on one such initiative, their employer’s comprehensive clinical trial program, in the on-demand 2020 ASAP BioPharma Conference session “Strategic Sponsor/Site Relationship-Based Approaches to Conducting Clinical Trials in the Changing Landscape of COVID-19,” and in the process revealed what may be the shape of pharma-CRO relationships to come.
How does a large organization like Genentech manage a long-term, one-to-many engagement with multiple clinical trial sites? Through rigorous relationship management and collaboration, said Fillos. The company operated many clinical trials at once for quite some time, and in the beginning, they were being run in silos. With each trial, scientific groups and functions from all over Genentech and its parent organization Roche would give input to partner sites on a variety of aspects of their respective projects. Within the last decade, Genentech figured out that it could reap larger collective benefits from these interactions if it could establish a top-down view of the entire portfolio. To capture collective trends and insights from all of these trials, alliance managers could function as information gatekeepers of sorts by serving as a central point of contact for both clinical trial partners and the rest of the Genentech organization.
“It can be quite complicated and disjointed in some cases in terms of communication to sites from different teams,” said Fillos.
Onboarding Processes and Startup Kits Get Trials Off on the Right Foot
This new collaborative network needed other staple alliance management practices, such as an onboarding process for new CROs, in which the parties establish mutual goals around capabilities, potential improvements, and performance targets. The topics and procedures pertaining to clinical trial execution dissected in these discussions, such as study design methods, standard-of-care issues, trial designs, disease landscapes, and treatment paradigms, can get very complex. However, in a long-term partnership model, partner sites get visibility into Genentech’s pipeline and thus have more time to address these issues and ready data management protocols in advance of launch dates.
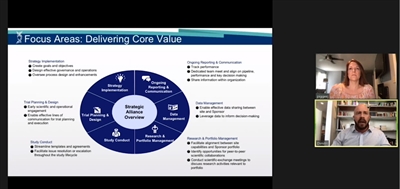
Fillos also outlined some of the broad ways the alliance team delivers core value to the clinical trial operation. It assists in actual trial planning and design by working with appropriate clinical-study personnel to map out efficient processes and communication protocols across multiple functions within Genentech and partner sites. The practice has also built out templates for master agreements, rate cards, and other aspects of clinical trials that have helped streamline operations. Fillos said Genentech now has a study startup kit for its organization and its participating CROs.
Cross-Function Junction: Forums, Scientific Exchanges Continually Improve Program
Alliance management also plays a big role in smoothing out disagreements that arise over time between parties by serving as the point of contact for initiating disputes in the escalation process. It has also taken on reporting responsibilities by tracking performance metrics and sharing data with internal Genentech and Roche cross-functional teams. Thanks to alliance management, the quality of clinical trial data gets improved and disseminated quicker than ever, and as a result, decisions get made faster in many situations, such as “when you need to look at data as quickly as possible to make a decision to escalate the dose in a phase 1 study,” said Fillos.
Perhaps most important, alliance management helps aggregate, apply, and share critical lessons gleaned from the organization’s entire portfolio of sites that you wouldn’t get from transactional CRO relationships. The alliance folks also foster valuable peer-to-peer collaboration by organizing “scientific exchange meetings,” in which parties from several research functions discuss key issues, such as how to write more efficient protocols or the impact a recent regulatory guidance might have on sites around the globe.
Genentech has also put together a cross-functional leadership forum, a meeting of executives who are “invested in building out long-term strategic site relationships to support the different areas within their functional groups,” explained Fillos. Forum members represent a wide swath of functions within the organization, such as regulatory, clinical science, bioanalytics, and biomarkers, among others. They examine high-level issues pertaining to risk mitigation, long-term relationship planning, qualitative and quantitative KPIs, and the portfolio’s overall capabilities and support programs.
“What are the site capabilities for the folks that we have been talking to and working with? Do they fit the needs of our pipeline?” said Fillos. “What are the trends of those molecules? We’re looking at therapeutic areas that are new. Are we going to need to look at patient populations that we haven’t looked at before?”
ARMing Sites and Sponsors with New Ways to Use the Network
Piggee delved deeper into the inner workings of the network model, which now encompasses more than 25 clinical trial sites worldwide. The bidirectional network consists of two pillars: 1) institution-sponsored research—clinical sites designed by partners with Genentech support—and 2) Genentech-sponsored trials that are devised in consultation with partners. A Scientific Alliance Relationship Manager (sARM) works with the first group to develop innovative institution-sponsored trials, while an Alliance Relationship Manager (ARM) focuses on improving trials that originate within Genentech. The sARM and the ARM work hand in hand to coordinate the two groups’ efforts.
The network’s governance structure was created to “ensure internal acceptance and utilization of the network across our teams. The last thing we wanted to do was build out this network and then not have teams utilize it. Obviously, that’s not ideal,” said Piggee.
A cross-functional team of executives from both Roche and Genentech make up an operational committee that monitors the network’s high-level objectives, which change over time. This committee oversees the aforementioned joint clinical operations forum.
A former sARM and ARM herself, Piggee has seen firsthand how this structure generates additional benefits through deeper engagement and collaboration that wouldn’t occur in transactional relationships.
“The direct link from site to sponsor, and vice versa, facilitates greater communication, timely transparency, rapid resolution of issues,” she said.
With both alliance managers advocating for the alliance, the sponsor and sites are in constant communication, but in a streamlined manner. The sARM and ARM serve as single points of contact for the two larger respective groups and ensure that input from third parties—CROs, vendors, other researchers, etc.—is incorporated without suffering “the downsides of having too many hands in the pot,” said Piggee. The alliance managers can also spot broader trends and issues developing across all two dozen–plus sites and make changes that will keep people utilizing the network.
Not Bad Company, More Like Cream: Supergroups Help Clinical Trial Execution Rise to the Top
The network model has spawned so much free-flowing interaction between all of the sites that the sites themselves are collaborating with one another on initiatives independent of Genentech—“an unexpected advantage” of the model, according to Piggee. This dialogue has encouraged candid feedback about the network itself. Genentech has created “supergroups” co-led by the sARM and ARM that are responsible for cultivating ideas that germinate from those discussions, creating strategies around them, and garnering organization-wide support. These supergroups have created:
- Comprehensive site capability profiles that help reduce duplicative site selection efforts,
- A line-of-sight tool that has increased transparency around future studies in the Genentech portfolio, and
- A process for gathering resources from the network to support outsourced studies.
As part of the network’s relentless focus on process and execution, Genentech instituted a formal operational review board. Historically, study sponsors have often solicited feedback from scientific experts on study protocols, but it hasn’t been common practice for them to seek out similar input on the actual execution of clinical trials. According to Piggee, real-world insight from patients and “the actual operational people doing these studies” helps mitigate protocol implementation challenges, unnecessary contract amendments and deviations, and other roadblocks that could potentially delay timelines. This review board has also instituted measures that have “decreased the burden of clinical trials on patients,” added Piggee.
Game-Changing Forums Help Sites Navigate COVID-19 Protocols
Like everyone else in the world, the network has had to pivot quickly in the face of COVID-19. It has begun to accelerate the adoption of emerging technologies, such as direct data transfer, telemedicine, e-consent, and remote monitoring, in response to limitations created by the pandemic. With the network’s help, sites have been able to make changes that have enabled them to continue oncology trials as COVID-19 continues to roil society. For example, some are now shipping oral drugs directly to patients. Once again, the single-point-of-contact structure has streamlined direct communication to the sites, which site management folks have appreciated—they don’t want a deluge of people reaching out to them about every minute alteration to trial operations.
Genentech instituted COVID-19 forums, from which sites could learn new processes by observing and interacting with other sites around the globe. Piggee called it a “game changer” for US sites to be able to monitor developments in Italy early on in the pandemic. Ongoing post-forum feedback has shed light on what has and hasn’t worked for other sites, helped sites compare methods for managing the crisis across countries, and ensured consistency in managing patients. With information changing fast, forum members found it useful to disseminate updates through mass network emails.
Fillos and Piggee provided much more background on Genentech’s novel clinical trial network, so if you registered for the 2020 ASAP BioPharma Conference and you are involved in early-stage clinical trials, don’t miss this opportunity to learn how to move away from the transactional clinical trial model. But hurry, the BioPharma Conference portal will only be open until Nov. 13!